A quarterly journal published by Intrepid Publishing, LLC. Frontiers of Legal Innovation (FLI) is a peer-reviews journal dedicated to disseminating significant results of scientific research that are comprehensive, groundbreaking and of fundamental importance to the scientific community. FLI has rigorous ethical standards. A prospective work published in this journal must exemplify these august tributes. The Author(s) must make compelling cases that it does. Journal articles will be published in a rolling format, so that when accepted for publication there will be a minimal time before the prospective article is published. When the journal is full (approximately ten articles or 100 pages upper limit per volume), then the next volume will be automatically opened.
Instructions to Authors
Manuscripts for consideration of publication must be submitted to the editorial board: [intrepidpublishing@gmail.com]
Executive Editor
Editorial Board
R. Izurieta
N. Poor
Two or more references will be solicited for each submission with the editorial board taking part in decisions as to acceptability for publication in this Journal.
Format for Submitted Manuscripts
Manuscripts to be considered for publication in FST must have the following format:
Evaluation of the Performance and Microbial Safety of the Solar Latrines
Monica Gray
*Department Physics and Engineering Science
Coastal Carolina University, South Carolina, USA
*Corresponding author. monicaannmariegray@gmail.com.
Article Info
Keywords:
solar, latrine, sanitation, biosolid, pathogen, pH, moisture and temperature
Abstract
About 1.2 million persons die annually as a result of diarrheal diseases which are typically caused or exacerbated by lack of adequate sanitation. Solar latrines offer an attractive alternative in areas of inadequate traditional sewerage infrastructure and resources. However, more research is essential to determine the ability of these systems to safely contain excreta for disposal and sanitize it for reuse. This study examined the effects of varying physiochemical parameters on pathogen reduction in 22 Tecpan prototype IV solar latrines, which were constructed as a part of a sanitation intervention by the El Salvador Ministry of Health in Vista Hermosa, El Majahual, El Salvador. Samples were collected from the solar vault and subsequently analyzed at days; 3, 7, 15, 30, 45, 60 and 75, starting three months after construction. Temperature, pH, moisture, and microbial content (Ascaris lumbricoides, Trichuris trichiura, hookworm, Clostridium perfringens, fecal and total coliform) were determined to ascertain the critical variables for, mechanism(s) and rate of pathogen reduction. Storage time and pH were determined to be the most significant variables, while desiccation, not thermal deactivation was the primary mechanism, for microbial inactivation. There was significant correlation (p = 0.007) between storage time and moisture content: biosolids were drier with successive samples. All latrines met U.S. Environmental Protection Agency (EPA) Class A Biosolid standard for helminthes (<1 ova/g) with as little as three days storage time. From the results, 59% of study latrines were able to achieve Class A status for fecal coliform at some point during the sampling period. The mixing of semi-dried and new biosolid complicated the determination of the rate of bacterial deactivation. Tecpan prototype IV was shown to be effective in microbial inactivation. However, to develop a more sustainable latrine, that is less dependent on additives for efficacy, further modifications such as increasing the vault temperature by solar concentration is necessary.
MAIN BODY OF MANUSCRIPT (Including the various sections of the paper):
INTRODUCTION
About 1.2 million people die annually because of diarrheal diseases, attributable to unsafe water, sanitation and hygiene, of which 99.8% occur in developing countries and most are children under age five (Clasen, et al., 2015). Most diarrheas are cause by pathogenic organisms such as viruses, bacteria, protozoa and parasitic worms, whose presence is as a result of and/or exacerbated by poor sanitation (Curtis and Cairncross, 2003; Curtis, Cairncross, and Yonli, 2000; Nadakavukaren, 2000).
Research has confirmed that at least twenty diarrhea causing microbes such as vibrio cholera reproduce inside human host and enter the environment via excreta (Curtis and Cairncross, 2003). Once in the environment, these pathogens can cause diarrhea in the same individual or a new host, mainly by the fecal-oral route (Curtis, Cairncross, and Yonli, 2000; Feachem, 1984; Khosla, Bhanot, and Karishma, 2005; Pruss-Ustun, Kay, Fewtrell, and Bartram, 2004). Therefore, promoting safe stool disposal is one of the most reliable methods of breaking this route of transmission (Curtis, Cairncross, and Yonli, 2000; Jinadu, Esmai, and Adegbenro, 2004; Khosla, Bhanot and Karishma, 2005; Woldemicael, 2000).
However, governments, especially those of developing countries where the need is greatest, have been slow or reluctant to implement sanitary interventions because of the prohibitive economical and ecological cost of seweraged sanitation systems (Khosla, Bhanot and Karishma, 2005; Pruss, Kay, Fewtrell, and Bartram, 2002). For lesser developed countries, the required physical infrastructure and water resources needed for contemporary “flush” toilets are generally nonexistent or insufficient to meet the demands of ubiquitous and rapidly growing populations, rendering their application unsustainable (Langergraber and Muellegger, 2005; Lundin, 1999; Winblad and Simpson-Hebert, 2004). On the other hand, pit latrines are an economically low cost, low maintenance disposal option. However, they are not feasible in areas with high population densities, high water tables, shallow bedrocks or sandy soils, because such systems may contaminate the surrounding area and groundwater, producing a hazard to public health and the environment (Esrey et al., 1998; Redlinger, Graham, Corella-Barud and Avita, 2001; Winblad and Simpson-Hebert, 2004).
Dry sanitation solves both these issues. These systems use composting and dehydration mechanisms to render the final product pathogen free, which is then removed and may be used as a soil conditioner (Peasey, 2000). Composting occurs when bacteria and other in situ organisms breakdown feces into its fundamental constituents (Redlinger, Graham, Corella-Barud and Avita, 2001). Urine is usually not diverted and, for optimal operation, temperature and airflow are carefully controlled and bulking material such as wood shaving is added (Peasey, 2000). Examples of composting toilets include; the SIRDO of Mexico and Carousel of the Pacific islands (Redlinger, Graham, Corella-Barud and Avita, 2001; Peasey, 2000). For dehydration toilets the urine is generally collected, diluted and used for watering crops. While lime, ash and/or earth are (is) added to the feces to act as desiccants and pH modifiers, thus reducing flies and eliminating odors (Jonsson, Stintzing, Vinneras and Salomon, 2005; Strauss and Bluementhal, 1990). If the temperature is sufficiently high, thermal deactivation may also occur. These include double-vault urine diverting toilets (DVUD) and the Tecpan solar toilets series of El Salvador (Gough, 1997; Moe and Izurieta, 2003).
For over 10 years, solar latrine designs have been evolving in El Salvador. The first design (Tecpan prototype I) was constructed in 1994 and consisted of a large single vault where fresh excreta were mixed with stored biosolid. One setback was that the solar panel (a black metallic cover) was often not positioned for optimum solar incidence. Modifications were made and a year later prototype II became operational, with an overall smaller vault that was partitioned to separate fresh and stored biosolid. Again, many of the solar vaults were not oriented for maximum solar exposure and had to be emptied every 15 days because of the reduced capacity. Prototype III was built in 1999 and had an overall larger, divided solar vault. This prototype consistently had lower bacterial levels compared to the two previous designs; however this was mostly attributable to the amount, and hence the effectiveness, of the desiccant used by the householders. For prototype IV the solar panel was designed with a 30o incline for increased incidence and the partitioned vault had a capacity to store biosolids up to 75 days (Fig. 1). In 2004, the first set of 22 Tecpan prototype IV solar latrines was built in El Majahaul, El Salvador.
The contents of the latrines’ solar vaults were tested for Ascaris lumbricoides, Trichuris trichiura, hookworm, Clostridium perfringens, fecal and total coliform, temperature, pH, and moisture content. A. lumbricoides, T. trichiura, hookworm and C. perfringens were chosen because they met the following criteria: their eggs and cysts are among the most persistent in the environment and they had a high incidence rate among study population. Fecal coliform was chosen as an indicator organism to help classify the end products according to U.S. Environmental Protection Agency (EPA) biosolid standards. Elevated pH and temperature, moisture removal and storage time are well accepted variables for the inactivation and destruction of pathogenic microbes (Esrey, Andersson, Hillers and Sawyer, 2001). The purpose of this paper was to examine the efficacy of the new solar latrine Tecpan prototype IV to destroy enteric pathogens by determining the critical variables for, and the rate and mechanism(s) of microbial reduction. Hence, with the establishment of the underlying principles governing pathogen die-off, better systems can be designed and subsequently constructed that will be able to endure some amount of homeowners’ neglect and still produce biosolids that meet recognized standards.
 FIG. 1. Cross-sectional elevation of a single-vault Tecpan prototype IV solar latrine showing the fresh pile, which is pushed down to the solar vault at 1-month intervals to produce the drying pile. The solar vault is separated into three sections by concrete blocks, to separate the already drying pile from incoming biosolid. The solar panel is positioned at a 30o angle over the drying pile for optimum solar incidence. Urine is diverted and is not mixed with the solids.
FIG. 1. Cross-sectional elevation of a single-vault Tecpan prototype IV solar latrine showing the fresh pile, which is pushed down to the solar vault at 1-month intervals to produce the drying pile. The solar vault is separated into three sections by concrete blocks, to separate the already drying pile from incoming biosolid. The solar panel is positioned at a 30o angle over the drying pile for optimum solar incidence. Urine is diverted and is not mixed with the solids.
MATERIALS AND METHODS
Study area. The study site was located in the Vista Hermosa community, El Majahual, within two miles of the Pacific coast of El Salvador. The community had sixty families, averaging six members per family, with most adults engaged in masonry or fishing for their livelihoods. Living conditions are poor, with most households lacking electricity and/or potable water. Vista Hermosa was chosen by the Ministry of Public Health for an intervention because the community was in urgent need of improved sanitation, a condition about which the entire community was quite vocal. A survey by the Ministry revealed that only 55% of the households had access to pit latrines and children frequently defecated in open fields. The area was dry with sparse vegetation making it ideal for solar latrine construction.
Field methods. Prior to sanitary intervention a baseline epidemiological and behavioral survey was administered to the study households, the results of which are detailed in Corrales, Izurieta and Moe (2006). To ensure that community members knew how to adequately maintain their solar latrines, health education training was offered through the Ministry’s local “Health Promoters”. Participants in this training program were instructed to push the excreta from the drop zone towards one of the three sections of the solar vault every month, to expose the excreta to solar radiation, and be desiccated and stored for inactivation. Households kept a log of when they began using the toilets and when fecal matter is moved to the solar vault. Sampling was postponed until 3 months after the latrines were built to allow for households to establish their patterns of using and maintaining the new toilets.
After this transitionary period, all study households were revisited and interviewed again before sampling began. Samples were taken from the top, middle and bottom of the pile in the solar vault (to form a composite sample) using a core device. The color and texture were noted and the temperatures inside and outside the vault were recorded at 3, 7, 15, 21, 30, 45, 60 and 75 days after the accumulated fresh pile was first pushed down to the solar vault. After final the final sample the biosolid was removed and used as a soil conditioner and the cycle is repeated by pushing new waste to the now empty section of the solar vault for processing.
Laboratory methods. Samples were promptly placed in a water and ice mixture and cooled to less than 4oC (EPA, 1994). For each latrine, equal portions of material from the different levels of the pile were mixed to form a composite sample, which was used in subsequent analyses. 50 g of composite material was placed in the oven at 103oC for 24 hours. The sample was then cooled in a desiccator and weighed continually until variation in the dried weight was <4%. Moisture content was then determined as a percentage of the wet weight. To determine sample pH, 20 g of previously air dried biosolid was mixed with 20 g of distilled water (Thomas, 1996).
100 g of the composite was suspended in buffer (10% suspension) and then siphoned off for each microbiological test. Total and fecal coliform and C. perfringens were quantified with multiple tube technique using A1 broth and iron milk media respectively. The Most Probable Number method (MPN) tables were used to generate estimates for these microbes (APHA, 1998). The presence of viable Ascaris, Trichuris and hookworm ova was assessed by an adaptation of the EPA Standard Methods (EPA, 1994).
Biosolid classification. The results from the analysis were classified according to treatment methods used by the households, established moisture ranges and EPA Standards for Class A and Class B. Treatment methods classification is given in Table 1 below. The percent moisture content was divided into five groups: <10, 10-20, 20-30, 30-40 and >40%. These groups were chosen to determine the optimum moisture content necessary for effective deactivation. To determine the actual inactivation mechanism, the MPN data was classified according to EPA standards for biosolid and compared with the moisture ranges described above (Redlinger, Graham, Corella-Barud and Avita, 2001).
Quality assurance and data analysis methods. Laboratory testing included routine instrument calibration with reagent standards, replicate tests, and the use of positive and negative controls. All data was double entered, and duplicate databases were compared for discrepancies. Data verification was conducted, including determination of outliers, identification of lost data and confirmation of logical inconsistencies. The data was then statistically analyzed using SYSTAT v. 10.2 (SYSTAT Software Inc, Richmond, CA).
RESULTS
Type of desiccant. Households typically used a combination of lime, ash and dirt as desiccation material, resulting in five methods; lime, lime and dirt, lime and ash, dirt, ash and lime and, dirt and ash (Table 1). Members used a handful of the desiccant of choice after each use. As a result, there was a wide distribution of points for relevant variables within and among household samples. This limited determination of valid relationships among study variables. To overcome this constraint, the data was categorized, see Table 1.
TABLE 1. Household Distribution among Treatment Methods
| Treatment Methods | Number of Household |
| Lime | 4 |
| Dirt, ash, lime (DAL) | 8 |
| Lime, dirt (LD) | 3 |
| Dirt, ash (DA) | 6 |
| Lime, ash (LA) | 1 |
| Total | 22 |
Qualitatively, the households as well as the method of desiccation had the most influence on the deactivation process. An Analysis of Variance (ANOVA) showed significant variation and interaction between these two factors (adj. R2 = 0.621; p-value < 0.001). Further study is needed to account for this phenomenon; the variability might be due to the fact that persons using the same method did so in an ad hoc manner, resulting in more variability within than between the samples.
Temperature. The temperature of the heap was measured by placing a thermometer in the middle of the heap. The temperature did not vary considerably (Fig. 2), but stayed within the range (26 – 39 oC). Fig. 3 shows that the temperature of the biosolid pile (average = 31.4oC) did not increase significantly above the ambient temperature (average = 29.4oC).
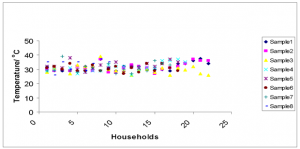 FIG. 2. Scatter plot showing the variation of the chamber temperature for each household at each sampling event.
FIG. 2. Scatter plot showing the variation of the chamber temperature for each household at each sampling event.
 FIG. 3. Scatter plot showing a comparison between ambient and chamber temperatures for all households at all sampling events.
FIG. 3. Scatter plot showing a comparison between ambient and chamber temperatures for all households at all sampling events.
pH. The pH of the study latrines ranged from pH 7.4 – 12.5, with the highest sample frequency occurring between pH 9.0 and pH 11.0 (Fig. 4). Overall, lime and dirt treatment method had the highest average pH (pH = 10.2).
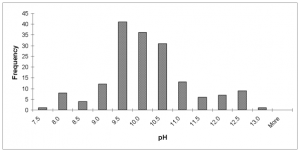 FIG. 4. Histogram showing the frequency distribution of the pH of solar vault biosolid over the entire sampling period.
FIG. 4. Histogram showing the frequency distribution of the pH of solar vault biosolid over the entire sampling period.
Moisture content. Overall, the moisture content ranged from 4% – 53%, with the highest number of samples occurring in the 30% – 34% intervals (Fig. 5).
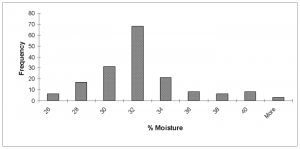 FIG. 5. Histogram showing the frequency distribution of the percent moisture content of solar vault biosolid over entire sampling period.
FIG. 5. Histogram showing the frequency distribution of the percent moisture content of solar vault biosolid over entire sampling period.
The moisture content was dependent on the storage time and the type of desiccant used. As expected, as storage time increased, moisture content generally decreased (Fig. 6).
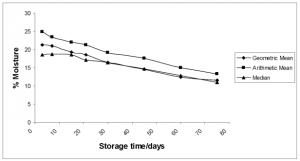 FIG. 6. Variation of percent moisture means with storage time over all sampling events.
FIG. 6. Variation of percent moisture means with storage time over all sampling events.
Samples with lower moisture contents were associated with the Dirt, ash and lime and the Dirt and ash treatment methods (Fig. 7).
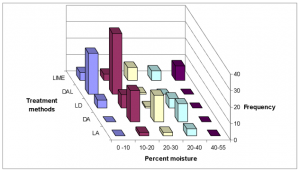 FIG. 7. 3-D histogram showing frequency of attaining established moisture ranges among treatment methods.
FIG. 7. 3-D histogram showing frequency of attaining established moisture ranges among treatment methods.
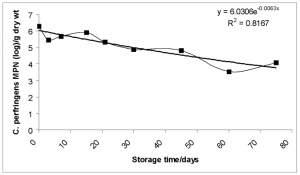
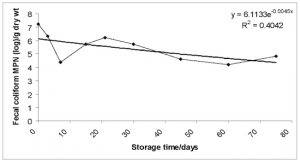
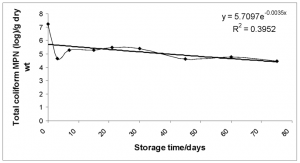
Biosolid microbes. No viable Ascaris, Trichuris or hookworm ova was found in all samples. Overall, as storage time increased the number of bacteria decreased with a slight increase after the 60 day sampling point (Figs. 8-10). This trend was generally common to individual households, with considerable variation between samples with microbial populations dropping levels as low as zero at various times during the sampling period. This was caused by new addition to the already drying pile.
FIG. 8-10. Showing the variation of the geometric mean of the concentration of C. perfringens, fecal and total coliform with storage time. Exponential trendlines were added with the corresponding equation and R2 value.
Effect of pH, storage time and moisture on microbial inactivation. Quantitatively, pH and length of storage were the most significant factors affecting inactivation (Table 2 and Eqn. 1).
EQUATION 1:
Where:
C = concentration of C. perfringens, Fecal coliform and Total coliform (MPN/g)
TABLE 2. Results for microbial inactivation with respect to storage and pH
| Microbes | â0 | â1 | â2 | p-value | Adj. R2 |
| C. perfringens | 13.47 | -0.782 | -0.031 | <0.001 | 0.334 |
| Fecal coliform | 15.77 | -1.296 | -0.026 | <0.001 | 0.361 |
| Total coliform | 8.238 | 0.323 | -0.008 | 0.005 | 0.049 |
In general, Dirt, ash and lime (DAL) addition generally showed the highest frequency of occurrence among all pH ranges (Fig.11) and the highest average pH (pH = 10.2). As a result, a greater number of latrines using this method achieved EPA Fecal Coliform Class A requirement (Fig. 12). However, this result may be skewed due to interference from the different number of users among methods (Table 1).
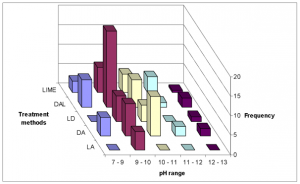 FIG.11. 3-D histogram showing frequency of attaining pH ranges among treatment methods.
FIG.11. 3-D histogram showing frequency of attaining pH ranges among treatment methods.
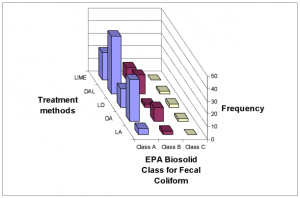 FIG.12. Frequency of achieving EPA Biosolids Fecal Coliform requirements for treatment methods
FIG.12. Frequency of achieving EPA Biosolids Fecal Coliform requirements for treatment methods
Of the two factors, pH seems to be the more significant, whereas storage time is more passive in its effect on the deactivation processes. Tables 3 and 4 show these results.
EQUATION 2:
TABLE 3. Regression results for microbial inactivation with respect to pH
| Microbes | â0 | â1 | p-value | Adj. R2 |
| C. perfringens | 11.705 | -0.70 | <0.001 | 0.153 |
| Fecal coliform | 13.7 | -1.16 | <0.001 | 0.273 |
| Total coliform | 7.87 | -0.31 | 0.008 | 0.04 |
EQUATION 3:
TABLE 4. Regression results for microbial inactivation with respect to storage time
| Microbes | â0 | â1 | p-value | Adj. R2 |
| C. perfringens | 5.79 | -0.03 | <0.001 | 0.142 |
| Fecal coliform | 3.15 | -0.015 | 0.047 | 0.026 |
| Total coliform | 5.051 | 0.01 | 0.197 | 0.005 |
Fig. 7 illustrates that treatment method Dirt, ash and lime (DAL) was associated with the lower moisture levels, ranges 0 – 10% and 10 – 20%. This method also had the highest frequency among pH ranges 9-11 and 12-13 (Fig. 11). Therefore, this treatment method had the highest frequency for achieving EPA Class A biosolids status (Fig. 12).
There is a significant correlation between storage time and moisture content (P =0.007). Fig. 6 shows an inverse relationship between these two parameters, while Figs. 8-10 illustrated a similar association between storage time and microbial concentration. Figs.13, 14 and 15 clarify the roles of storage time and moisture on microbial deactivation.
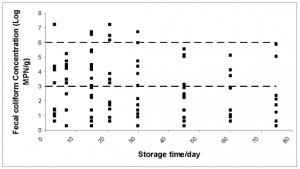 FIG. 13. Scatter plot showing that as storage time increase more samples achieve higher grade status. This show that storage time is a critical variable.
FIG. 13. Scatter plot showing that as storage time increase more samples achieve higher grade status. This show that storage time is a critical variable.
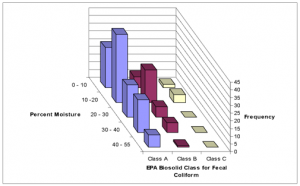 FIG.14. 3-D histogram showing the frequency of achieving EPA standard for fecal coliform for established moisture ranges. This shows that desiccation is the primary mechanism for microbial inactivation.
FIG.14. 3-D histogram showing the frequency of achieving EPA standard for fecal coliform for established moisture ranges. This shows that desiccation is the primary mechanism for microbial inactivation.
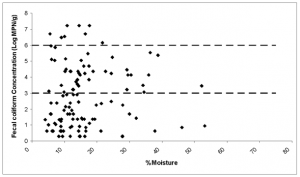 FIG.15. Scatter plot illustrating numerically the information from Fig. 14.
FIG.15. Scatter plot illustrating numerically the information from Fig. 14.
DISCUSSION
In this study, in order to determine the safety of handling and disposing of biosolid from the Tecpan prototype IV solar latrines, a wide range of representative microbes were studied. These included soil transmitted helminthes (STH) such as A. lumbricoides, T. trichiura and hookworm; and bacteria (C. perfringens, fecal and total coliform). These are ideal as indicator organisms to predict the reduction of resistant pathogens, covering both egg and spore forming microbes.
The overall objective was to determine if this new prototype shows an improvement over previous versions in its ability to produce high quality biosolids. In order to accomplish this, critical variables and the primary mechanisms associated with the rate of pathogen reduction were determined. The study variables included temperature, pH, storage time and moisture content. Mechanisms associated with this type of latrine were thermal deactivation and desiccation.
Temperature was not found to be a significant factor. From the literature thermal deactivation of Ascaris is effective at temperatures above 50oC (Juris, Planchy, Toth and Venglosvsky, 1992; Winbald and Simpson-Hebert, 2004). The average temperature was 31.4oC. Therefore thermal deactivation was not found to be a critical factor for microbial die-off. This is similar to the average temperatures found in the last three prototypes (30oC).
Factors pH and storage time played the most important role in deactivating microorganisms in fecal material. The highest frequency of EPA Class A biosolid occurred in households that used a mixture of Dirt, ash and lime (DAL) as desiccant, which also had the highest average pH (10.2). This is not much higher than the average pH for prototypes I-III (average pH =9.9). As for the previous versions, the efficacy of the latrine was more due to the type and amount of desiccant used rather than thermal deactivation and moisture removal due to solar irradiation.
The average storage time for this prototype was 60 days compared to 26 days for the previous versions. While storage time was found to be significant, it had a more passive effect on the microbial die-off process. That is, it facilitated the drying process as was seen from the correlation between storage time and moisture content (P =0.007). From the result, microbial concentration decreased with decreasing moisture content. However, for desiccation to be the dominant mechanism, the average moisture content must fall below 5%, which did not occur (Gotaas, 1956).
The rate of pathogen reduction could not be determined due to the considerable variation of microbial concentration among samples. This was caused by new addition to the already drying pile. No viable Ascaris, Trichuris or hookworm ova was found. It is uncertain whether this resulted from the effectiveness of the latrines or medication administered during the baseline epidemiological study. From these results the end products fully met EPA’s Class A biosolid Standards for protozoa < 1 ova/g. While only 59% of households achieve this status for Fecal Coliform < 1000 MPN/g. Additional study is required to evaluate if the resulting biosolids is appropriate for agricultural use.
To ensure that the EPA’s Class A Standards are met, a storage time longer than 75 days and a separation of newly deposited fecal matter from the semi-sanitized product are required. According to the trendline equation from Fig. 9, under these conditions, it requires over one-year storage time for the biosolid to achieve Class A status for fecal coliform.
These preliminary results are limited and do not adequately indicate that prototype IV solar latrine showed improvement in its capacity to inactivate pathogenic microbes over its predecessors. Solar latrines have a lot of potential but a greater understanding of microbial die-off mechanisms occurring in them is required. It is true that the dried biosolids have great environmental and health advantages, but so far there is no guarantee that the resulting product can meet the EPA’s Class A biosolid standards.
Finally, the efficacy of the latrines was more attributable to the type and amount of desiccant used rather than solar incidence and the subsequent elevation in temperature. To ensure sustainability, it is necessary to develop the solar vault that facilitates an increase in temperature and can utilize the sanitizing power of UV radiation from the sun. More research and further modifications are therefore needed. To reduce the variability and bias and to improve the design the following are recommended:
- Ensuring that the amount and/or type of desiccant are (is) fixed. This will create an idealized scenario; however, this will ensure a better understanding of the inactivation mechanisms.
- Proper use is very important for these toilets to function well. It is recommended that new material not be added to older product.
ACKNOWLEDGEMENTS
This work was supported by the El Salvador Ministry of Public Health and UNICEF in El Salvador. Special thanks to Drs. Ricardo Izurieta and Noreen Poor of the College of Public Health, University of South Florida, Tampa, Florida 33620
REFERENCES
- American Public Health Association (APHA). Standard methods for the examination of water and wastewater, 20th ed. American Public Health Association, Washington, D.C.
- Black, R., S. S. Morris, and J. Bryce. Where and why are 10 million children dying every year? The Lancet 361: 2226-2234.
- Corrales, L., R. Izurieta, and C. In Press. The association between intestinal infections and type of sanitation system in rural El Salvador. Journal of Tropical Medicine and International Health.
- Curtis, V., and S. Cairncross. Effect of washing hands with soap on diarrhea risk in the community: a systematic review. The Lancet Infect Dis. 3: 275-281. http://infection.thelancet.com.
- Curtis, V., S. Cairncross, and R. Yonli. Review: Domestic hygiene and diarrhea – pinpointing the problem. Tropical Medicine and International Health 5: 22-32.
- Clasen TF, Alexander KT, Sinclair D, Boisson S, Peletz R, Chang HH, Majorin F, Cairncross S. 2015. Interventions to improve water quality for preventing diarrhoea. Cochrane Database of Systematic Reviews. Issue 10. Art. No.: CD004794. DOI: 10.1002/14651858.CD004794.pub3
- Esrey, S. A., I. Andersson, A. Hillers, and R. Sawyer. Closing the Loop: Ecological Sanitation for Food Security. Publication on Water Resources, No.18. Swedish International Development Cooperation Agency (SIDA)
- Esrey, Steven A., J. Gough, D. Rapaport, R. Sawyer, M. Simpson- Herbert, J. Vargas, and U. Winblad. Ecological Sanitation. Swedish International Development Co-operation Agency.
- Feachem, R.G. Interventions for the Control of Diarrheal Diseases among Young Children: Promotion of Personal and Domestic Hygiene. Bulletin of the World Health Organization 62:467-476.
- Gotaas, H. 1956. Composting: Sanitary disposal and reclamation of organic wastes. World Health Organization, Geneva.
- Gough, J. El Salvador experience with dry sanitation. In Ecological Alternatives in Sanitation. Proceedings from SIDA Sanitation Workshop. Balingsholm, Sweden. 6-9 August 1997.
- Jinadu M., O. A. Esmai, and C. A. Adegbenro. Disposal of children’s faeces and implications for the control of childhood diarrhea. Journal of The Royal Society For The Promotion Of Health 124: 276-279.
- Jonsson, H., R. Stintzing, B. Vinneras, and E. Salomon. Guidelines on Use of Urine and Faeces in Crop Production. 3rd International symposium on ecological sanitation, May 2005, South Africa.
- Juris, P., P. Plachy, F. Toth and, J. Venglovsky. Effect of biofermentation of pig slurry on Ascaris suum eggs. Helminthologia 29:155-159.
- Khosla, R., A. Bhanot, and S. Karishma. Sanitation: A Call on Resources for Promoting Urban Child Health. Indian Pediatrics: Environmental Health Project 42:1199-1206.
- Langergraber, G., and E. Muellegger. Ecological Sanitation – A way to solve global sanitation problems? Environment International 31:433-444.
- Lundin, M. Assessment of the Environmental Sustainability of Urban Water Systems. Department of Technical Environmental Planning, Chalmers University of Technology, Sweden.
- Moe, C.L., and R. Izurieta. Longitudinal study of double vault urine diverting toilets and solar toilets in El Salvador. 2nd International symposium on ecological sanitation, April 2003, Luebeck.
- Morris, K. “Silent Emergency” of Poor Water and Sanitation. The Lancet 363:954.
- Nadakavukaren, A. Our Global Environment: A Health Perspective. Waveland Press, Inc, Illinois.
- Peasey, A. Health Aspects of Dry Sanitation with Waste Reuse. London: Task No. 324, Water and Environmental Health at London and Loughborough (WELL).
- Prüss, A., K. David, L. Fewtrell, and J. Bartram. Estimating the Burden of Disease from Water, Sanitation, and Hygiene at a Global Level. Environmental Health Perspectives 110:537-542.
- Pruss-Ustun, A., D. Kay, L. Fewtrell, and J. Bartram. Unsafe Water, Sanitation and Hygiene. In WHO, Comparative Quantification of Health Risks for Environmental and Occupational Risk Factors, Switzerland.
- Redlinger, T., J. Graham, V. Corella-Barud, and R. Avitia. Survival of Fecal Coliform in Dry-Composting Toilets. Applied and Environmental Microbiology. 67:4036-4040.
- Strauss, M., and U. J. Blumenthal. Use of Human Wastes in Agriculture and Aquaculture: Utilization Practices and Health Perspectives. Dubendorf, International Reference Center for Waste Disposal. Report No. 08/90.
- Thomas, G.W. Soil pH and soil acidity. In Methods of soil analysis: chemical methods, part 3. Soil science society of America, Madison, Wisconsin.
- U. Environmental Protection Agency (EPA). 1994. A plain English guide to the EPA part 503 biosolids rule. Office of Wastewater Management, U.S. Environmental Protection Agency, Washington, D.C.
- World Health Organization (WHO). Water, Sanitation and Hygiene Links to Health: Facts and Figures. General Comment No.15 (2004). World Health Report 2000. Geneva. http://www.who.int/whr/2004/en/report04_en.pdf.
- Winblad, U., and M. Simpson-Hébert. Ecological sanitation – Revised and Enlarged Edition. Stockholm, Sweden: Stockholm Environmental Institute.
- Woldemicael, G. The effects of Water Supply and Sanitation on Childhood Mortality in Urban Eritrea. J. Biosoc. Sci. 32:207-227.
Publication Charges
Page Charges – $10/page
Reprints:
30 Articles: $50
75 Articles: $85
100 Articles: $100
We will make a maximum of 100 reprints / articles.
Upon acceptance for publication, a bill for page changes along with an order form for reprints will be sent to the contact person that is indicated during the submission process. All payments must be received before an article can be published.
Certified checks made payable to ‘Intrepid Publishing’ are acceptable, as is payment via PayPal.
(Note: To pay by PayPal, log onto PayPal.com, the select ‘Send Money’ and enter ‘intrepidpublishing@gmail.com’ into the recipient’s address field, and confirm payment by clicking ‘Send’.